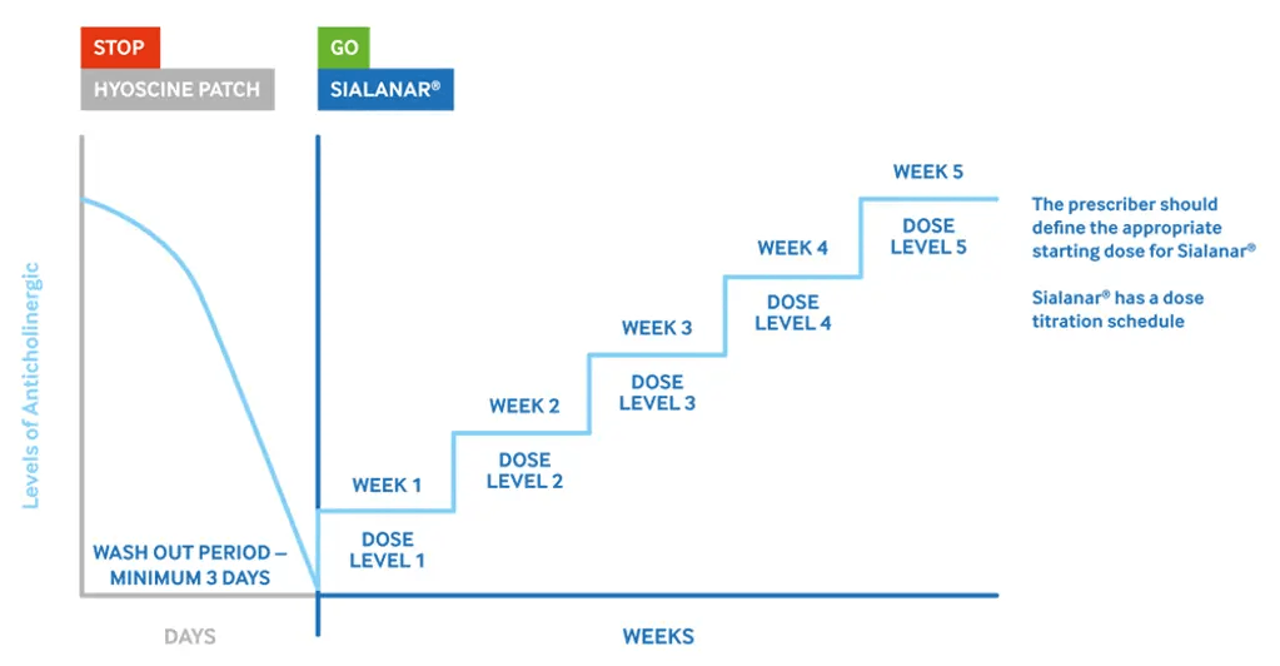
Care should be taken when moving from another type of anticholinergic drug due to the potential for increased risk of anticholinergic adverse effects.1 There is no available data to support transferring patients from a non-glycopyrronium bromide anticholinergic (e.g. hyoscine/scopolamine or atropine) to Sialanar®.1,2
A washout period from the first drug should be considered. Sialanar® should be prescribed following the dose titration table, starting from dose level 1 to balance efficacy and side effects.2
The half-life of free scopolamine is approx. 10 hours so the drug should be cleared within about 72 hours. However, the ultimate decision as to when to start the second drug and the appropriate starting dose should lie with the treating clinician.2
From 1mg/5ml glycopyrronium bromide oral solutions:
Oral preparations of glycopyrronium bromide are not interchangeable on a microgram-for-microgram basis due to differences in bioavailability. Care should be taken if switching between oral preparations and dosing adjusted accordingly.3
The document linked below provides guidance on the equivalent doses of 1mg/5ml glycopyrronium bromide oral solutions4-6 and Sialanar® (2mg/5ml glycopyrronium bromide) oral solution1, due to differences in concentration and bioavailability.

Clinical Evidence
Glycopyrronium has demonstrated lower rates of side effects7 and treatment cessation7,8 vs. hyoscine.



