
Tailored titration for flexible and accurate dosing1
This content is intended for UK & ROI healthcare professionals. Not a healthcare professional? Return to audience selector.
It has raspberry flavouring, a titration schedule based upon patient’s weight and is suitable for administration orally or via feeding tubes.1


Tailored titration for flexible and accurate dosing1

60% less volume vs.1mg/5ml glycopyrronium oral solutions, helping to improve patient acceptability and tolerability1-6

Minimal excipients to reduce toxicity risk - sugar, alcohol and sorbitol free 1,6,7,8


Watch the excipients animation
Sialanar® is designed for children, using sucralose as a sweetener instead of sorbitol1 and is suitable for patients on a ketogenic diet (<5mg/ml carbohydrate)1,11
Sialorrhoea can cause significant clinical problems and negatively impact quality of life for patients and carers12,13
Physical consequences
Posterior sialorrhoea: can lead to aspiration, increasing the risk of respiratory infections, hospitalisations and mortality14-16
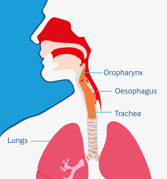
Management of sialorrhoea is recommended to reduce risk factors for aspiration, helping to prevent further respiratory illness15
Psychosocial concerns

Visit the resources section to find product support materials for patients/carers and educational resources for healthcare professionals.

Head to this section to find out more about the dosing and titration schedule for Sialanar®.

Glycopyrronium has demonstrated lower rates of problematic side effects18 and treatment cessation18,19 vs. hyoscine.
UK-SIA-25-0062 | October 2025
Adverse Event Reporting Information
UK: Adverse events should be reported. Reporting forms and information can be found at: www.mhra.gov.uk/yellowcard.
Republic of Ireland: Adverse events should be reported. Reporting forms and information can be found at: www.hpra.ie.
Adverse events should also be reported to Proveca Limited. Phone: +44 333 200 1866 E-Mail: medinfo@proveca.com